How It Works
From Ingredient to Substantiation in Four Steps
What used to take months of manual research now happens in minutes.
Search
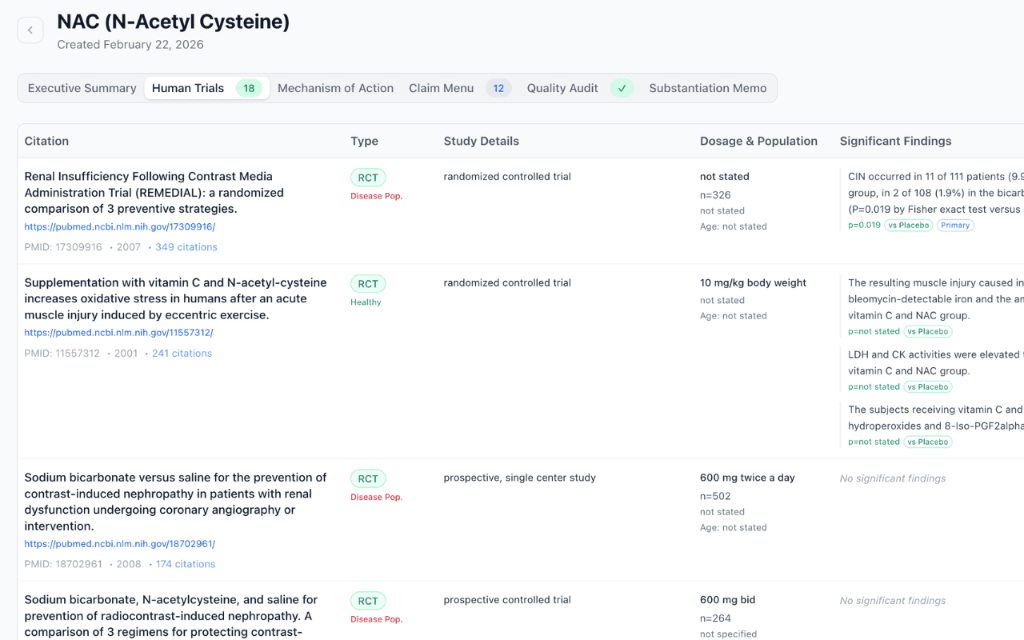
Enter your ingredient and Nutra Comp searches PubMed for relevant clinical trials — RCTs, meta-analyses, and systematic reviews on healthy human subjects.
Analyse
AI reviews each study for oral administration, subject health status, significance of findings, and extracts p-values, effect sizes, and conclusions.
Generate
Automatically produces FDA-compliant structure–function claims ranked by evidence strength, plus a complete substantiation memo ready for review.
Export
Download your clinical evidence report, claims, and substantiation memo as polished, print-ready PDFs to share with your legal and compliance teams.
Features
Everything You Need to Substantiate with Confidence
Built for supplement brand owners who need rigorous, compliant evidence — without the compliance consultants.
PubMed Clinical Trial Search
Automated, exhaustive search of PubMed for RCTs, meta-analyses, and systematic reviews on healthy human subjects with oral administration.
AI-Powered Evidence Analysis
Intelligent extraction of p-values, effect sizes, study populations, and significance levels — distinguishing across-group from within-group findings.
FDA-Compliant Claim Generation
Structure–function claims generated in strict compliance with FDA, Amazon, and Google Merchant Center requirements. No disease claims, ever.
Substantiation Memo Builder
Produces a comprehensive substantiation memo with confidence scores, evidence tables, and mechanism-of-action data — ready for internal review.
One-Click PDF Export
Export your entire clinical evidence report, generated claims, and substantiation memo as professional, print-ready PDF documents.
Brand Profile Management
Save your brand details, ingredient lists, and past reports in one place. Revisit and update substantiation as new research emerges.
Pricing
Simple, Transparent Pricing
Choose the plan that fits your portfolio. All plans include full substantiation reports and compliance-ready documentation.
Standard
5 ingredient searches/month
- 5 ingredient searches per month
- Full clinical evidence reports
- Structure–function claim generation
- Substantiation memo generation
- PDF export
Pro
25 ingredient searches/month
- 25 ingredient searches per month
- Full clinical evidence reports
- Structure–function claim generation
- Substantiation memo generation
- PDF export
- Priority processing
Enterprise
Unlimited ingredient searches/month
- Unlimited ingredient searches
- Full clinical evidence reports
- Structure–function claim generation
- Substantiation memo generation
- PDF export
- Priority processing
- Dedicated support
FAQ
Frequently Asked Questions
Everything you need to know about Nutra Comp and supplement substantiation.